Back to elementary, Homogeneous and Heterogeneous are mentioned... both of these are Solution and Colloid. If you can't figure what which is Heterogeneous or Homogeneous for Solution, Homogeneous is Solution the remaining is for Colloid.
Let's define again what is Colloid and Solution.
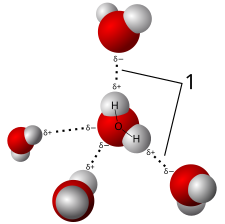
Colloid — mixture of solid particles that are suspended within the liquid.
Solution — mixture of solid particles that are completely dissolved within the liquid.
The only difference between Colloid and Solution is the properties of solid particles within the liquid.
Let's define again what is Colloid and Solution.
Colloid — mixture of solid particles that are suspended within the liquid.
Solution — mixture of solid particles that are completely dissolved within the liquid.
The only difference between Colloid and Solution is the properties of solid particles within the liquid.